











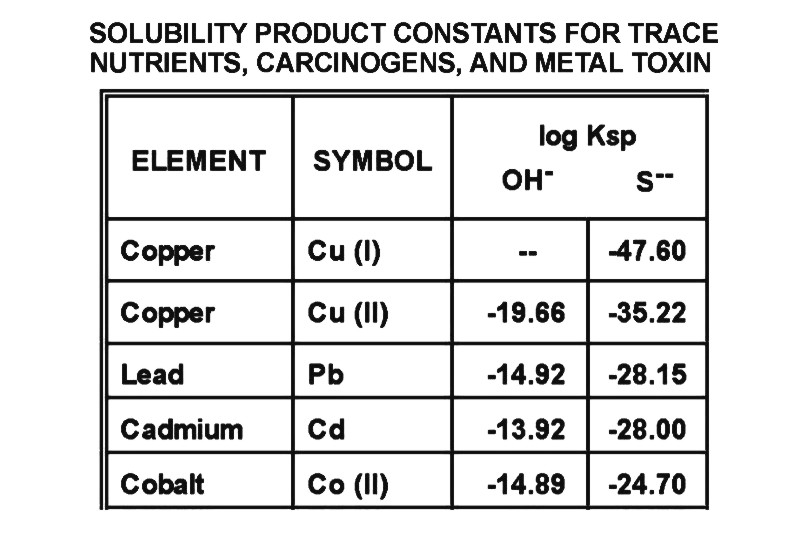
Different oxidation states of the same toxic metal will bind sulfides with different tenacities. Thus, reduction with antioxidants can be beneficial, since diminishing binding tenacity can be helpful, but when metals are aggressively reduced to the plus one state, they can bind more tightly than in their higher oxidation states. Thus, it is not a good idea as a rule to use more powerful chemical reductants, and it is probably far safer to stick with those antioxidants occurring in our natural environment, food and drink. For example, most elements can have a variety of oxidation states, iron being far more toxic in its plus three or (FeIII) state. Tin (IV), Cobalt (III), and Chromium (VI) are other examples of metals that are far more toxic in their higher oxidation states..